Respiratory
Voiant is the imaging partner of choice for BICR in respiratory imaging biomarkers.
Forged through the union of three best-in-class clinical trial leaders, Voiant combines deep respiratory expertise with advanced AI technology to deliver high-quality imaging data for pulmonary trials. Our platform supports rapid eligibility assessments by expert readers, with turnaround times accelerated by AI-powered QC and real-time site feedback. Backed by seamless third-party integrations and strategic partnerships, Voiant enables fast, reliable, and cost-effective imaging delivery.
AI-Powered Excellence in
Respiratory Imaging Technology
Voiant Hub 2.0 is purpose-built to meet the evolving demands of respiratory trials, integrating AI across key stages of the imaging workflow. These enhanced capabilities improve the consistency of lung disease assessments and enable earlier insights into pulmonary pathology through advanced imaging biomarkers. This empowers sponsors to make more informed decisions, adapt trial strategies in real time, and accelerate the development of therapies for patients with respiratory conditions.
Automated lobe segmentation enables precise, region-specific quantification for advanced metrics such as QILD, QLF, and air trapping.
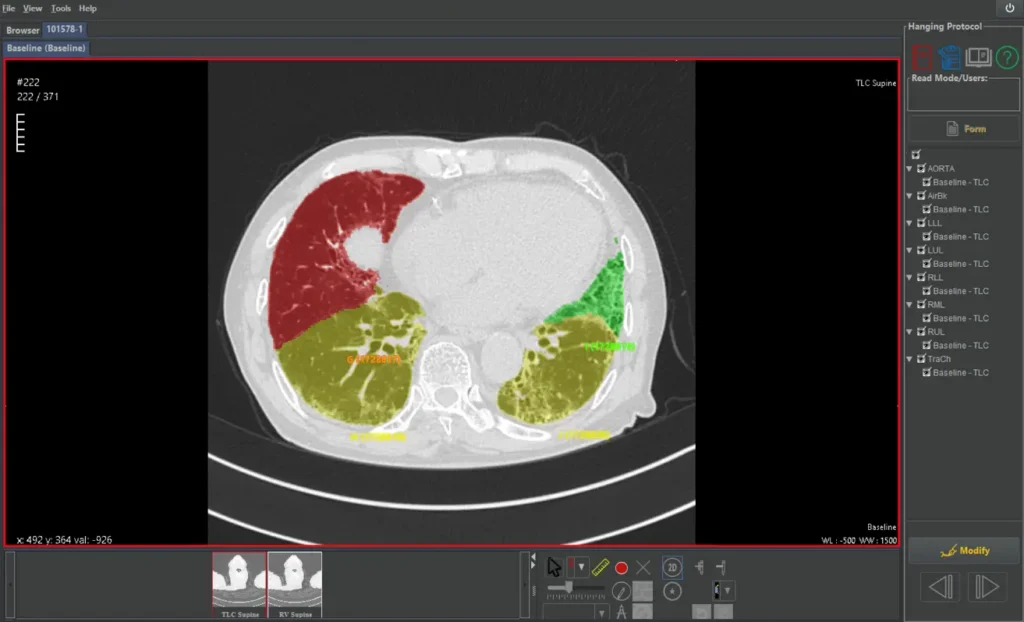
Quantitative Image Analysis (QIA) combine proprietary denoising with CT-based quantification of fibrotic lung disease, including lung volume and QILD metrics, to support precise, pattern-based evaluation of interstitial scarring.
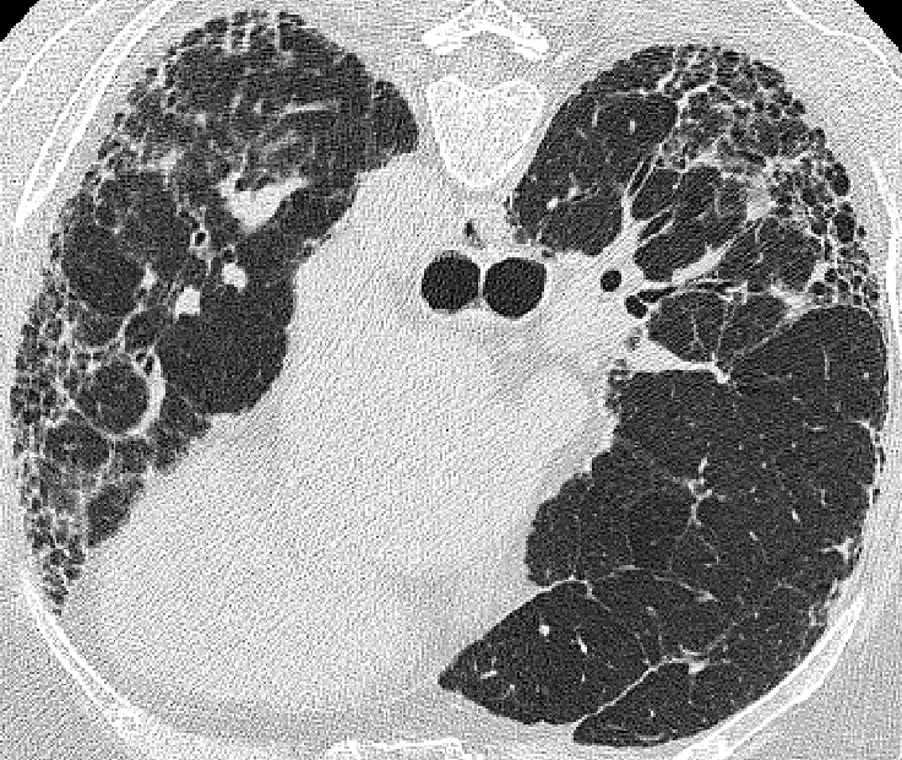

Through our partnership with Thirona, we deliver advanced respiratory image quantification, including bronchial tree analysis and precise, non-invasive vascular analysis, to support more accurate and sensitive phenotyping of pulmonary disease(s) and evaluation of treatment efficacy.
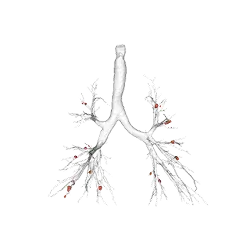
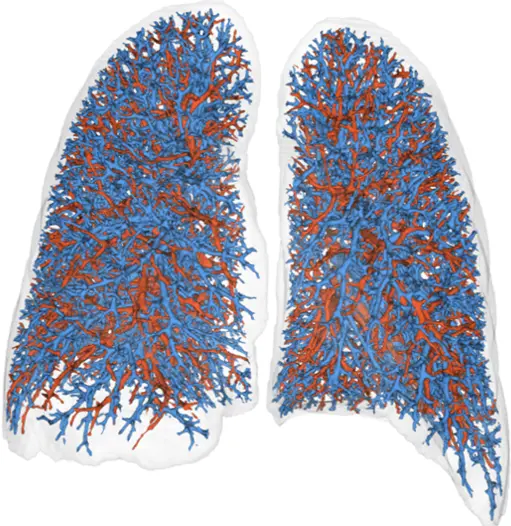

Respiratory Imaging Expertise
Voiant brings deep therapeutic insight and operational excellence to Phase I–IV respiratory trials, including pivotal studies. Our experience spans a wide range of respiratory indications and advanced imaging biomarkers, supported by KOL-led read paradigms and seamlessly integrated through our AI-powered platform.
Experience Across Respiratory Indications
Developed by KOL-led read paradigms, including ATS/ERS and Fleischner Society criteria, our extensive clinical and regulatory expertise enables us to deliver imaging services across multiple obstructive and fibrotic lung diseases through every phase:
- Interstitial Lung Diseases (ILD)-associated conditions, including:
- Idiopathic Pulmonary Fibrosis (IPF)
- Progressive Pulmonary Fibrosis (PPF)
- Systemic Sclerosis
- Other Connective Tissue Diseases
- Chronic Obstructive Pulmonary Disease (COPD)
- Emphysema
- Cystic Fibrosis
- Bronchiectasis
- Pulmonary Hypertension
- Sinusitis
- Lung Cancer
- COVID-19
- Sarcoidosis
Expanding Biomarker Capabilities
Powered by Voiant Hub 2.0, our platform leverages AI-driven image analysis and seamless third-party integration to deliver advanced biomarker capabilities with consistent, high-quality reads. Through strategic collaborations, we tailor imaging solutions to meet evolving biomarker needs and drive meaningful outcomes for patients and sponsors alike.
- Regional Lung Volumetrics
- Lung Density
- Quantitative Interstitial Lung Disease (QILD):
- Lung Fibrosis (QLF)
- Honeycombing (QHC)
- Ground Glass Opacity (GGO)
- Airway Analysis, including LungQ® Bronchial-Artery (BA), wall thickening, and mucus plugging assessments*
- Pulmonary Vascular Analysis, including LungQ®-AVX for pulmonary artery-vein phenotyping*
- Weighted Reticulovascular Score (WRVS)*
* Biomarkers available in collaboration with our partners – Voiant AI-Lab’s agile platform enables rapid integration of new paradigms, often in a matter of weeks, making our capabilities limitless.
Comprehensive Breath Holding
Precise breath hold execution is not just a procedural detail – it is foundational to the integrity of respiratory imaging. In trials where subtle changes in lung structure or function serve as sensitive biomarkers, even minor inconsistencies in breath hold can introduce variability that compromises data accuracy and reproducibility.
Our comprehensive breath hold training ensures that every image is captured under standardized conditions, minimizing motion artifacts and enhancing signal consistency across timepoints and sites. This level of control is essential for detecting early disease progression, evaluating therapeutic response, and supporting regulatory-grade endpoints in respiratory trials.

The Voiant
Respiratory Imaging Team
Our respiratory imaging team brings together subspecialty-trained radiologists, pulmonologists, and technicians that are extensively trained and certified through Voiant’s rigorous internal programs and comprehensive breath hold training. Guided by in-house Subject Matter Experts (SMEs), we collaborate with sponsors to design imaging protocols, optimize workflows, and maintain continuous quality control to deliver accurate, reproducible results in a cost-effective manner.
Your imaging partner from start to endpoint
Learn more about how Voiant’s innovative solutions and expert services can provide custom-tailored support for your next trial.