Oncology
Voiant is the leading clinical imaging partner for oncology trials.
Formed through the powerful union of three best-in-class clinical imaging companies, Voiant combines deep therapeutic knowledge with advanced imaging endpoint technology to deliver the high-quality data required for regulatory success. Our expert team, AI-powered platform, and global network of accredited sites ensure rapid, consistent, and reliable imaging delivery. With over 350 oncology trials under our belt, Voiant is the trusted partner for BICR and clinical trial success.
AI-Powered Excellence in
Oncology Imaging Technology
Voiant Hub 2.0 is designed to meet the evolving demands of oncology trials by leveraging AI into key aspects of the imaging workflow. These enhanced capabilities not only improve the reliability of response assessments but also drive earlier insights into tumor biology through imaging biomarkers. As a result, sponsors can make more confident treatment decisions, adapt trial strategies in real time, and ultimately bring therapies to patients faster.
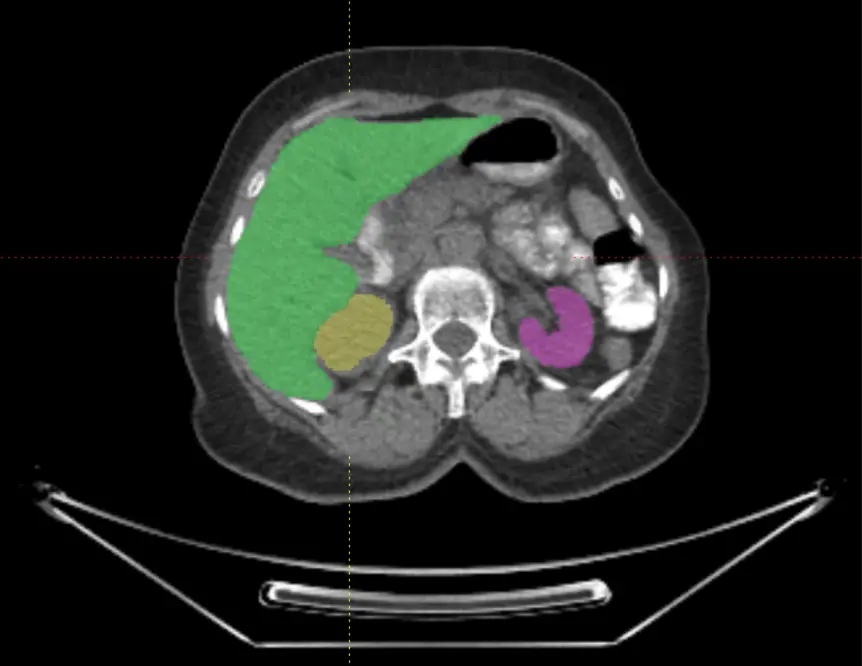
Voiant Hub 2.0 streamlines segmentation and tracking for semi-automated RECIST and volumetric automated RANO assessments, enhancing accuracy, consistency, and compliance in tumor response and tumor burden evaluation.
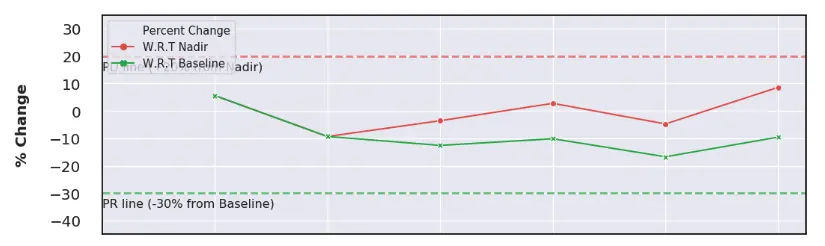
RECIST and RANO spider plots offer comprehensive insights into drug efficacy by clearly evaluating patient responses to treatments.
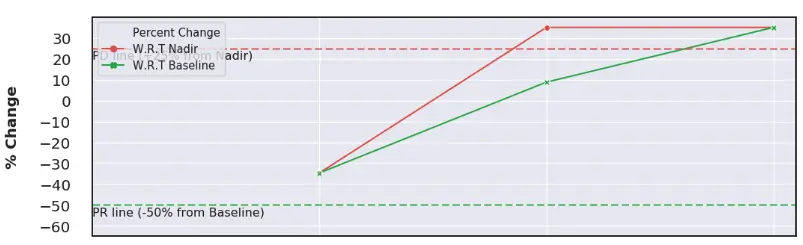
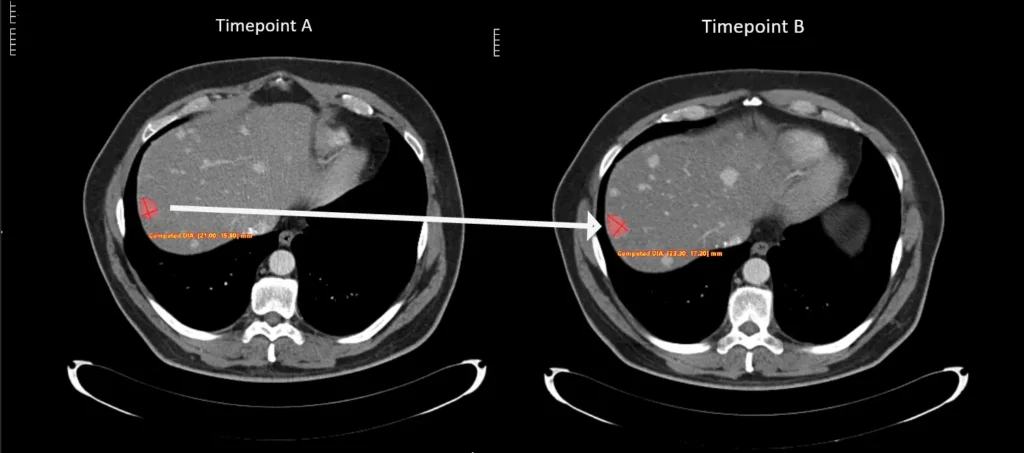
These interactive tools visually track lesion changes across multiple timepoints, offering a clear, longitudinal view of disease progression and treatment response over time.

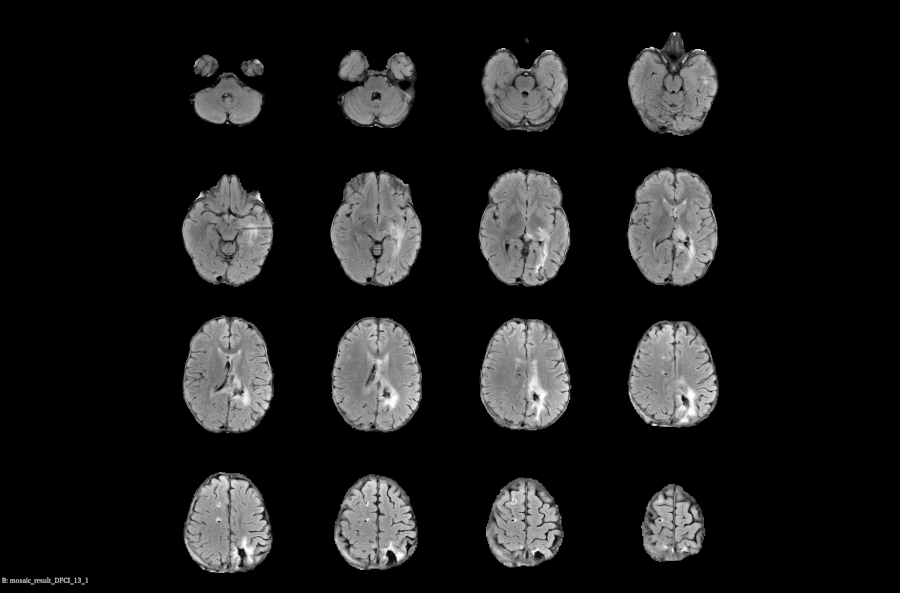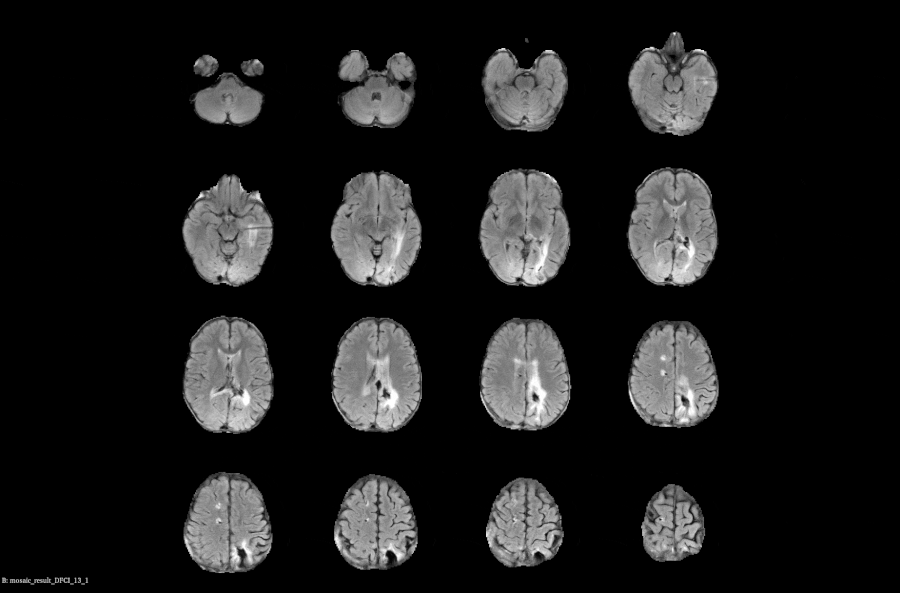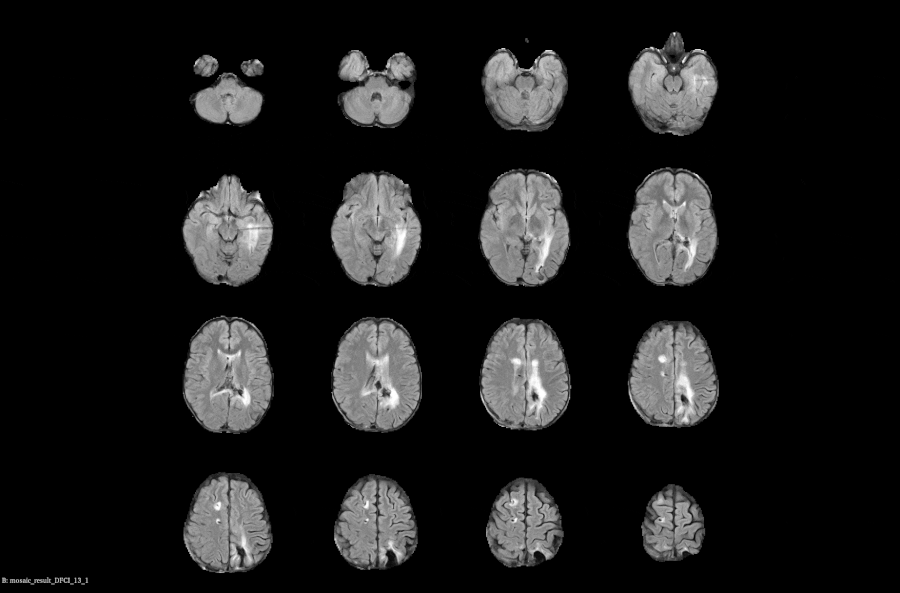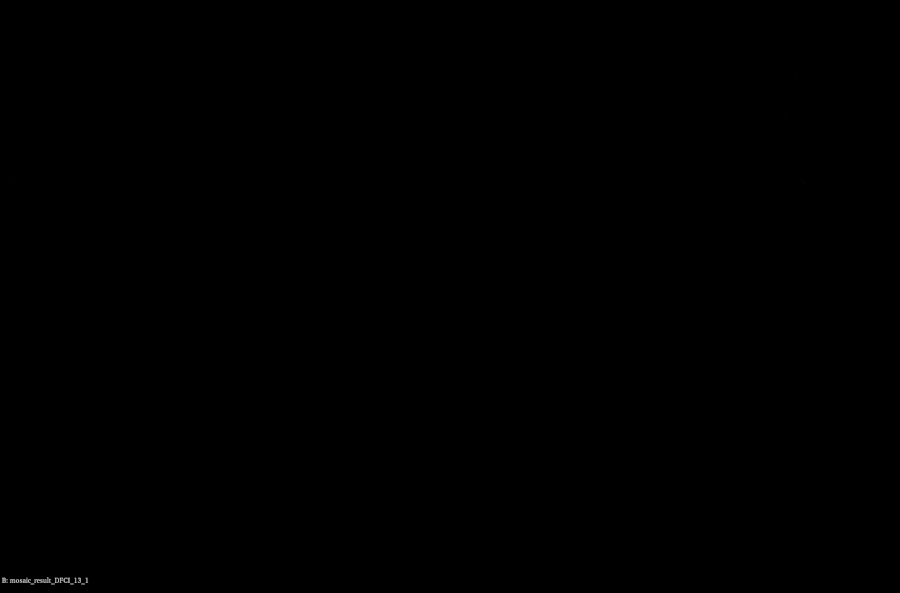
Real-time Compliance Checks: Automatically monitor and flag imaging data with protocol parameters to prevent delays and ensure data quality
Subject Assessment History: Centralized, time-aligned view of each subject’s prior reads and imaging data to support longitudinal evaluations
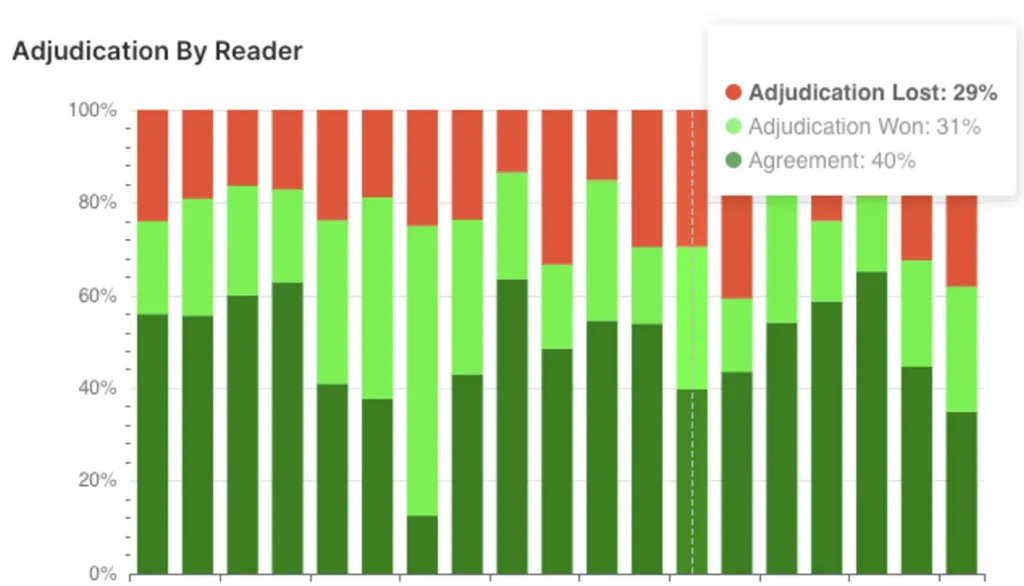
Oncology Imaging Expertise
Voiant brings deep therapeutic insight and operational excellence to Phase I–IV oncology trials, including pivotal studies. We combined scientific expertise with AI-powered solutions to enhance imaging assessments—such as semi-automated RECIST and RANO—while supporting a broad range of tumor types, evaluation criteria, and imaging biomarkers with clarity and confidence.
Experience Across Oncology Indications
Our team has delivered imaging CRO services for oncology trials across a wide range of indications including:
- Acute Lymphocytic Leukemia (ALL)
- Acute Myeloid Leukemia (AML)
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Chronic Lymphocytic Leukemia (CLL)
- Colon Cancer
- Colorectal Cancer (CRC)
- Endometrial Cancer
- Gastric Cancer
- Glioma: HGG, LGG
- Head and Neck Cancer
- Hepatocellular Carcinoma
- Liver Cancer
- Lymphoma
- Melanoma
- Multiple Myeloma
- Neuroblastoma
- Non-small and Small Cell Lung Cancer (NSCLC, SCLC)
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Renal Cell Carcinoma (RCC)
- Bone Metastasis
- Sarcomas
- Cutaneous Squamous Cell Carcinoma
- Thyroid Cancer
- Urothelial Cancer
- Uveal Melanoma
Evaluation Criteria Expertise
Voiant has extensive experience across various type of evaluations criteria, including standard reads such as RECIST and RANO and their latest modified variants, as well as immuno-oncology criteria across skin and other solid tumor indications:
- RECIST 1.1, iRECIST, irRECIST, mRECIST
- RANO; mRANO, RANObm; RANO 2.0
- PCWG2/PCWG3
- Tumor Volumetrics
- Cheson 1999, 2007
- Choi
- FDG and Novel Radiotracer Quantitative and Semiquantitative Assessments; TLG, MTV
- IWG
- INRC
- LungRADS
- LYRIC
- Macdonald
- Oncology Over Read
- PCSNL
- WHO
Expanding Biomarker Capabilities
Powered by Voiant Hub 2.0, our platform captures early biological signals and enables advanced, volumetric tumor characterization—unlocking a growing portfolio of imaging biomarkers that refine trial eligibility, accelerate exploratory endpoints, and drive targeted, image-informed strategies:
Radiomics
- Volume
- Shape and Contour Features
- Intensity Features
- Texture Features
- Lesion Heterogeneity
- Rate of Lesion Size Change
Additional MRI
- ADC
- Blood Volume
- Perfusion and Permeability
- T2 & Fat Water Mapping
Additional PET
- Dynamic SUV Measures
- Total Lesion Glycolysis
- Metabolic Tumor Volume

Ocular Safety Services
Systemic oncology drugs can be associated with serious ocular side effects — from dry eye and blurred vision to retinal damage — making early detection and monitoring critical. At Voiant, we bring deep expertise in eye health to help sponsors assess ocular toxicities with precision and confidence. Our robust Ocular Safety Study package enables seamless integration of ocular safety into trials, supporting the development of safer, more effective therapies. With our white-glove support, sponsors receive high-quality data and smooth execution from start to finish.
Our package includes:
- Comprehensive, hands-on support for sites new to ophthalmic imaging through every step of the process
- Protocol consultation with highly qualified medical experts for ocular safety assessment
- Accelerated site certification for ophthalmologic image collection
- Rapid analysis and safety reports
- Guidance and support with regulatory agencies
Your imaging partner from start to endpoint
Learn more about how Voiant’s innovative solutions and expert services can provide custom-tailored support for your next trial.